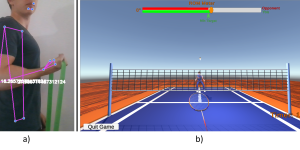
REHYB@SK: The ReHyb project held the final workshop in Bad Aibling, Germany
June 10, 2024 – Bad Aibling, Germany – The ReHyb project partners concluded their final workshop in Bad Aibling, Germany at Schoen Klinik. In the workshop, there was a participation …